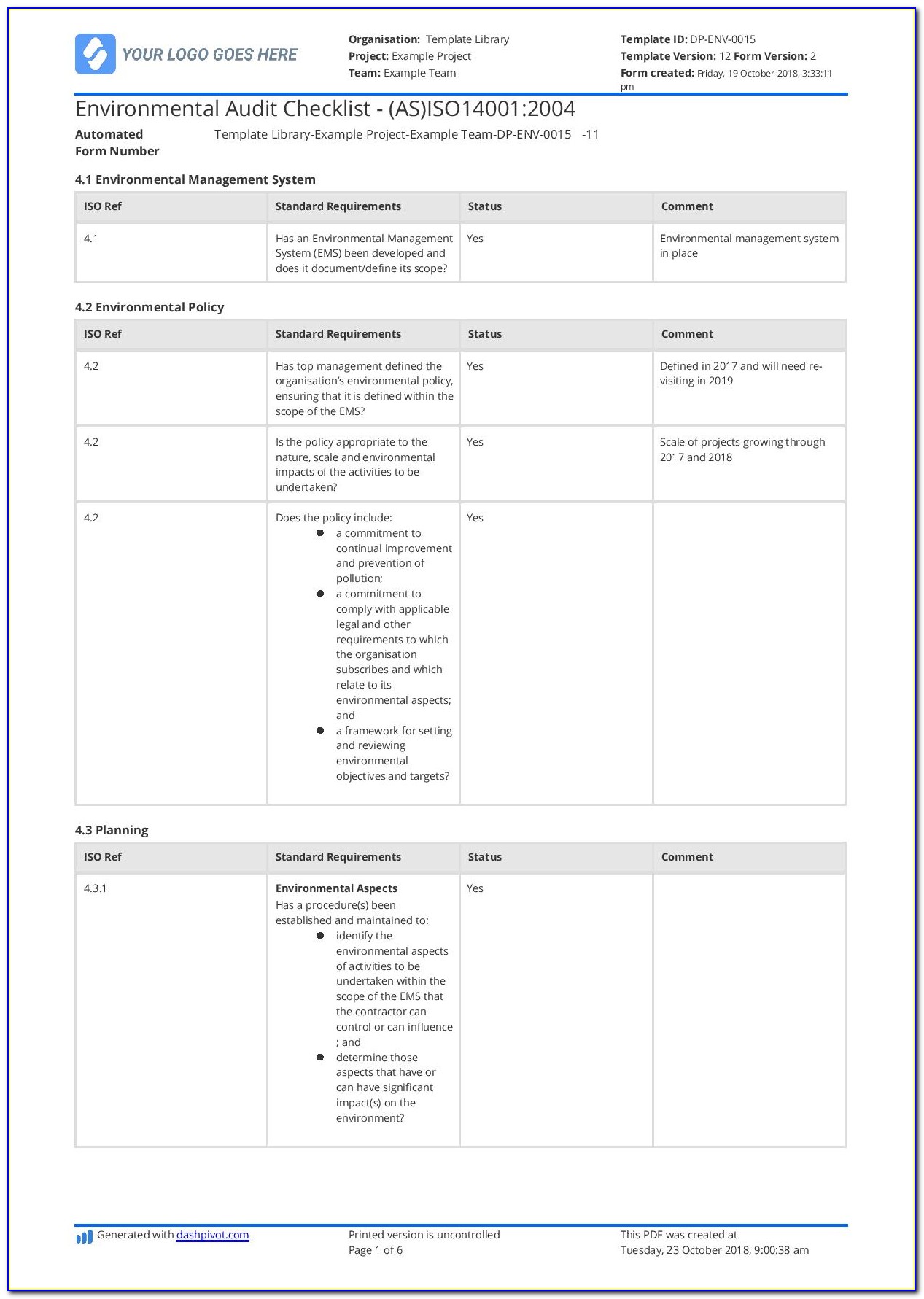
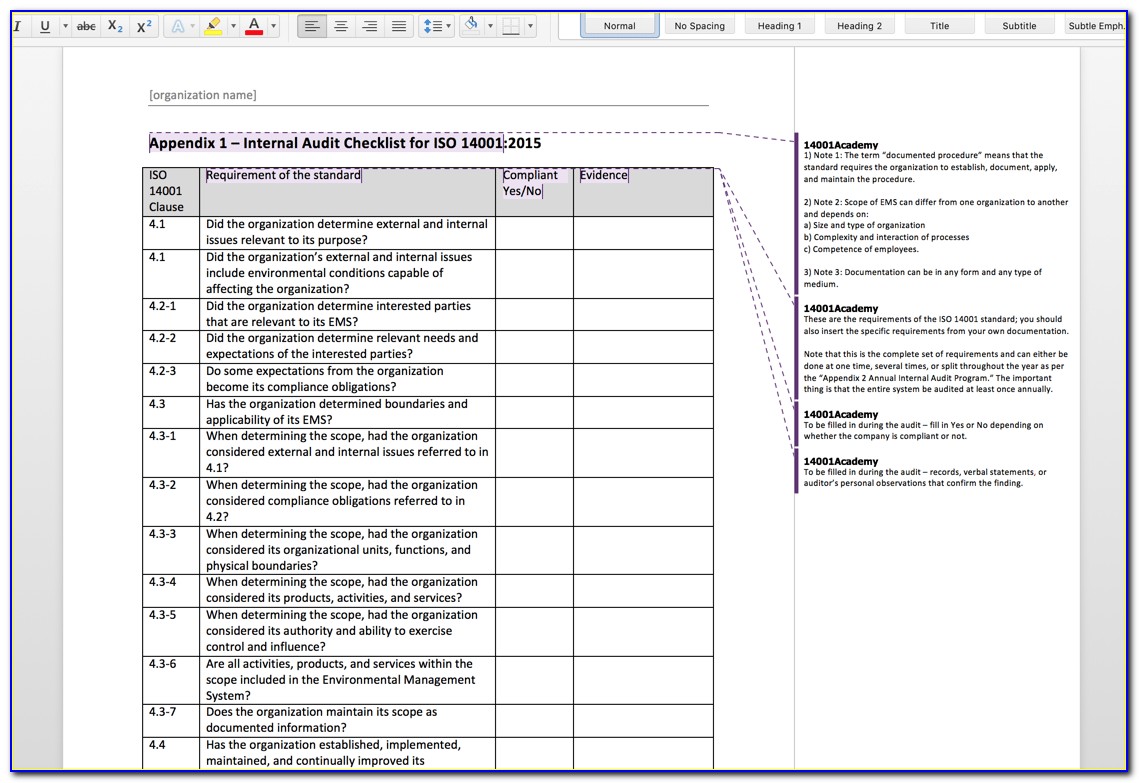
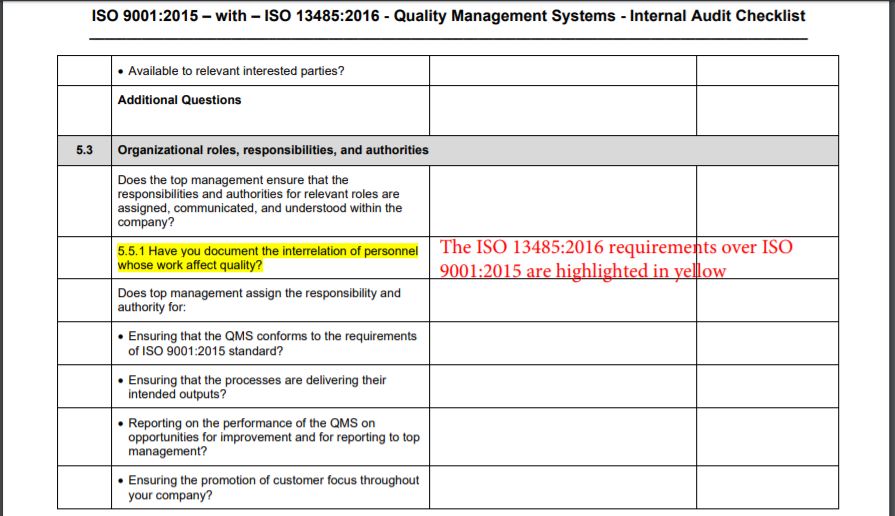
Iso 13485 Audit Checklist Template - Helps to identify process gaps. Web save time with a mobile app that generates comprehensive and insightful iso 13485 reports as you finish an audit. Web download an iso 13485 risk management plan template. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed? Web forms and checklists are used to record data, capture information, and facilitate compliance with iso 13485 requirements. For the example above, the audit checklist could include questions on supplier evaluation, and a review of the supplier audit reports that have been collected, to see if they. Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was uncovered. Web by qualitymeddev apr 5, 2021 internal audit internal audits are one of the most important process within a quality management system for medical device manufacturers and having an iso 13485 audit checklist is an essential tool that could be used to prepare and manage the internal audit process. The downloadable iso 13485 audit checklist contains twelve tabs that begin. Web objective parties conduct internal audits.
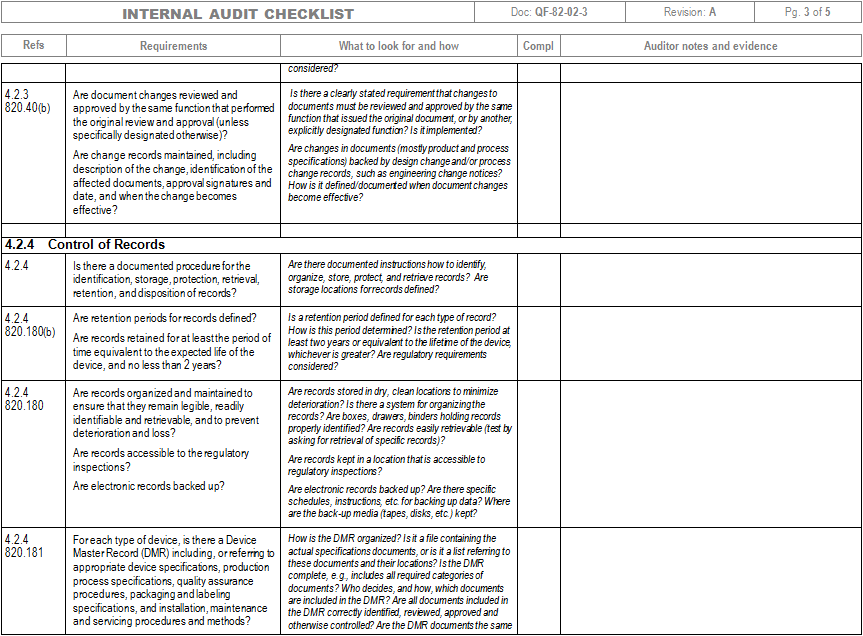
IMSXpress ISO 13485 and FDA QSR 21 CFR Part 820 Internal Audit
Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Web • november 18, 2022 • meg sinclair the 4 best iso 13485 audit checklists an iso 13485 audit can make even the most seasoned medical device quality managers bite their nails. Web by qualitymeddev apr 5, 2021 internal audit internal audits are.
ISO 13485 2016 Internal Auditor Checklist TQS Inc.
Free white paper that provides guidelines for each clause of the iso 13485 standard. It helps assess whether a company is ready to undergo an iso 13485:2016 certification audit by a third party. Helps to identify process gaps. Web save time with a mobile app that generates comprehensive and insightful iso 13485 reports as you finish an audit. The documentation.
Iso 13485 Audit Report Template
Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Web objective parties conduct internal audits. Oliver eidel the iso 13485 is the standard for quality management in the medical device industry. Web updated june 22, 2023 iso 13485 templates dr. Web the checklist is created by reviewing the iso 13485:2016 standard and.
IMSXpress ISO 13485 and FDA QSR 21 CFR Part 820 Internal Audit
Download as pdf downloaded 2717 times ★ ★ ★ ★. Web save time with a mobile app that generates comprehensive and insightful iso 13485 reports as you finish an audit. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Web the checklist is created by reviewing the iso 13485:2016 standard and any.
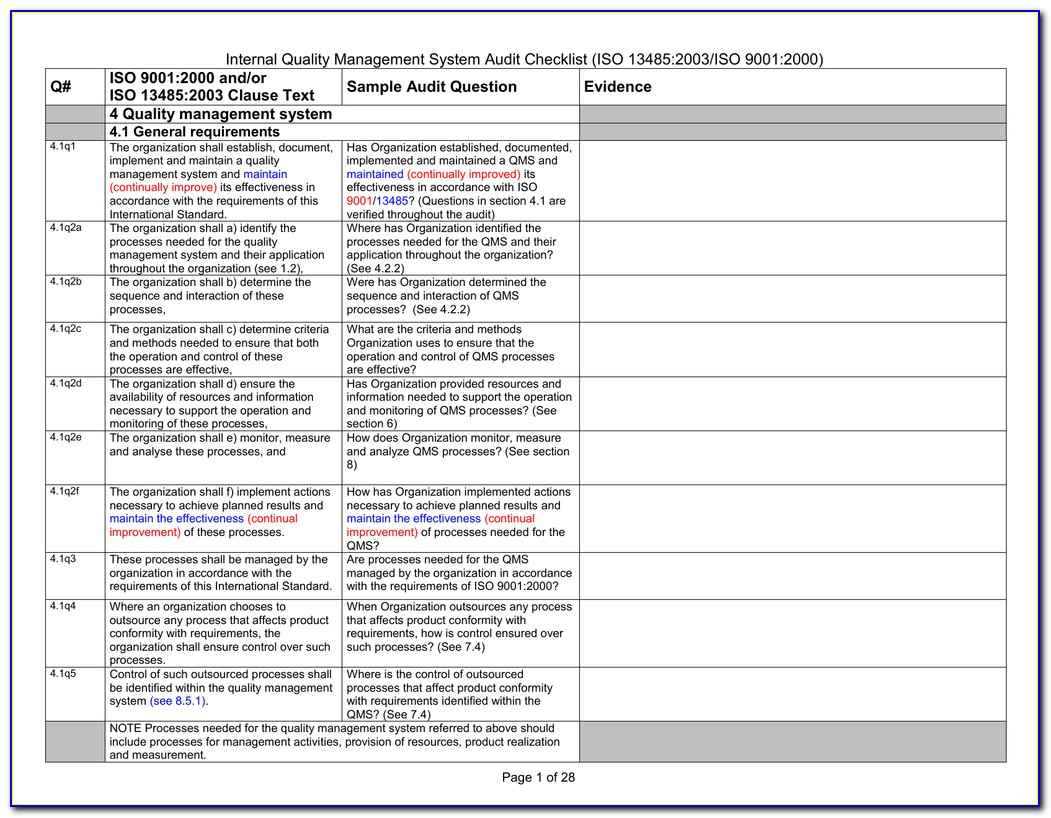
Iso 13485 internal audit checklist dadfat
Web the checklist also has other uses, such as. Preview a sample iso 13485 pdf report here. Web the iso 13485 audit checklist contains a series of questions and status updates to ensure that everything matches the plans defined in the organization’s qms regarding internal activities, supplier evaluation, and an evaluation of the supplier audit reports. Web save time with.
Internal Audit Report Template Iso 10 10 Lessons I've Learned From
The quality manual defines the scope of your qms and its procedures within your qms and describes the interaction of processes within your qms. Web updated june 22, 2023 iso 13485 templates dr. Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was uncovered. The standard includes 77 clauses, so there.
Internal Audit Checklist Template Iso 13485
Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was uncovered. Here are all our posts on this standard, and also all questions our consulting clients have asked us. Web the checklist is created by reviewing the iso 13485:2016 standard and any documented procedures or undocumented processes for the activity to.
FDA QSR & ISO 134852016 QMS Internal Audit Checklist Free Download
Web the checklist also has other uses, such as. Free white paper that provides guidelines for each clause of the iso 13485 standard. Use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. The checklist is best used by trained and practicing auditors to evaluate or assess.
Iso 13485 internal audit checklist blisslasopa
Web the checklist also has other uses, such as. Web save time with a mobile app that generates comprehensive and insightful iso 13485 reports as you finish an audit. The standard includes 77 clauses, so there are a lot of ways to fall short—even if you're working with the most comprehensive of iso 13485 audit checklists. Upon completion, the audit.
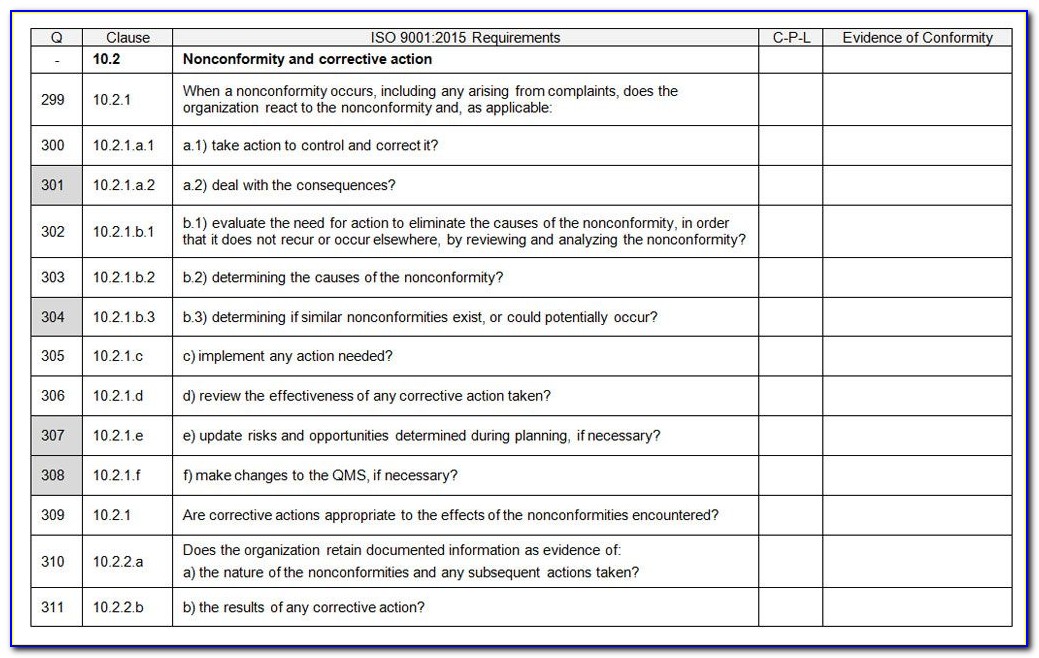
ISO 90012015 to 134852016 Internal Audit Checklist ISO 13485 Store
An internal audit assessment is a formal, comprehensive comparison of the current state of an organization’s processes and procedures against a standard or regulation. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was.
Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the iso 13485:2016 standard. The downloadable iso 13485 audit checklist contains twelve tabs that begin. Helps to identify process gaps. Save the iso 13485 template online and automatically share reports with members of the organization through formats such as weblink, pdf, word, or csv. Web the iso 13485 audit checklist contains a series of questions and status updates to ensure that everything matches the plans defined in the organization’s qms regarding internal activities, supplier evaluation, and an evaluation of the supplier audit reports. The documentation template may be used for iso 13485 certification audit purposes. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was uncovered. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out. Use this iso 13485 internal audit checklist template to determine whether the company's quality management system (qms) is compliant with the iso standards. Web the checklist also has other uses, such as. For the example above, the audit checklist could include questions on supplier evaluation, and a review of the supplier audit reports that have been collected, to see if they. Preview a sample iso 13485 pdf report here. Web updated june 22, 2023 iso 13485 templates dr. The standard includes 77 clauses, so there are a lot of ways to fall short—even if you're working with the most comprehensive of iso 13485 audit checklists. Here are all our posts on this standard, and also all questions our consulting clients have asked us. Web download an iso 13485 risk management plan template. Web • november 18, 2022 • meg sinclair the 4 best iso 13485 audit checklists an iso 13485 audit can make even the most seasoned medical device quality managers bite their nails. Web the checklist is created by reviewing the iso 13485:2016 standard and any documented procedures or undocumented processes for the activity to determine what should happen. Web looking to make your own or download iso 13485 audit checklist template to view all the tasks required and tick off the tasks when completed?
Web Forms And Checklists Are Used To Record Data, Capture Information, And Facilitate Compliance With Iso 13485 Requirements.
Web be ready for qms inspections with an iso 13485 audit checklist. The documentation template may be used for iso 13485 certification audit purposes. Here are all our posts on this standard, and also all questions our consulting clients have asked us. Web updated june 22, 2023 iso 13485 templates dr.
Web Save Time With A Mobile App That Generates Comprehensive And Insightful Iso 13485 Reports As You Finish An Audit.
Web objective parties conduct internal audits. Web the checklist also has other uses, such as. It helps assess whether a company is ready to undergo an iso 13485:2016 certification audit by a third party. Document templates contain an average of twenty comments each, and offer clear guidance for filling them out.
Web The Checklist Is Created By Reviewing The Iso 13485:2016 Standard And Any Documented Procedures Or Undocumented Processes For The Activity To Determine What Should Happen.
The quality manual defines the scope of your qms and its procedures within your qms and describes the interaction of processes within your qms. Upon completion, the audit checklist helps the auditor review to reconfirm if any aspect of the evaluation process was uncovered. Web the iso 13485 audit checklist contains a series of questions and status updates to ensure that everything matches the plans defined in the organization’s qms regarding internal activities, supplier evaluation, and an evaluation of the supplier audit reports. Validation, monitoring, inspection and test defined and documented for all stages of product.
An Internal Audit Assessment Is A Formal, Comprehensive Comparison Of The Current State Of An Organization’s Processes And Procedures Against A Standard Or Regulation.
Web an iso 13485 audit checklist is used by quality managers to determine whether the company's quality management system (qms) is compliant with the iso 13485:2016 standard. Web the documentation template may be used for iso 13485 certification audit purposes. Web the documentation template may be used for iso 13485 certification audit purposes. Preview a sample iso 13485 pdf report here.